Computational system for identification of variant proteins using mass spectrometry
INTRODUCTION:
Variation of biological systems is highly informative. In medicine, variation points out altered processes and directs selection of therapies. In forensic science, for instance, genetic variants establish identity and enable tests of paternity. Recent developments enable use of mass spectrometry of proteome for identification of variants.
TECHNOLOGY (INVENTION) DESCRIPTION:
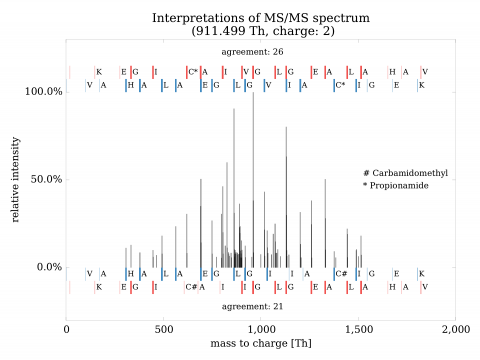
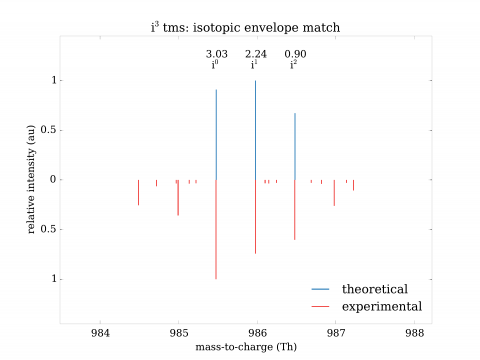
Identification of variants in mass spectrometric data poses significant challenges. Regular procedures yield high rate of false findings (as high as 95% being incorrect). To resolve the situation, we have developed both a mathematical theory of identification and high-performance system implementing it. The approach has shown precise, selective identification of variant proteins and also rare events in general. The invention can be used for identification of mutant and polymorphic proteins (e.g., for personalized medicine), rare events (e.g., post-translational modifications), establishment of genetic relationship and strong quality control of samples.
ADVANTAGES OVER EXISTING SOLUTIONS:
The advantages can be compared to a) nucleotide-based solutions such as DNA/RNA sequencing or b) other solutions in proteomics. In the former case, the approach has the advantage of direct observation of expressed proteins, their various forms and quantities. In the latter case, the solutions in proteomics either do not exist at all, or are marginal and without large-scale validation. Validations turned out to be critical and have shown need for extensive development of both mathematical and computational aspects of identification.
DEVELOPMENT STATUS (STAGE):
Large-scale validation (≈ 11 TB), including cancer cell lines, patients and xenografts. Access through web interface.
PUBLICATIONS:
IP PROTECTION STATUS:
TECHNOLOGY / IP OWNERS :
Palacky University Olomouc — Institute of Molecular and Translational Medicine (IMTM), Faculty of Medicine and Dentistry.
More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)