Diagnostic kit for HPV detection
INTRODUCTION:
Cervical cancer is the seventh most common malignancy with mortality rate of 6.8 per 100,000 people. It is caused by the presence of aggressive forms of HPV (human papilloma virus). More than 2.6 billion woman are at the risk of the cancer and about 500.000 cases are annually detected worldwide. The technology allows the sensitive detection of the
TECHNOLOGY (INVENTION) DESCRIPTION:
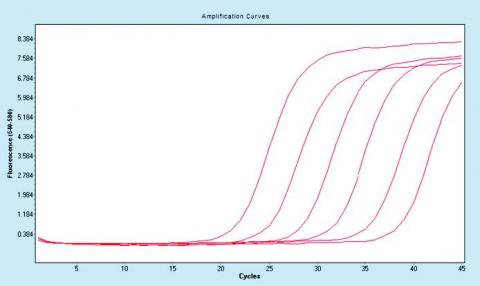
The principle of the technology is based on a quantitative fl uorescent detection of amplifi cation of HPV genes E2, E6 using the polymerase chain reaction (PCR) in real time using specifi c TaqMan probes. The technology detects 3 genes (HPV E2, E6, and human GAPDH) in one PCR reaction and contains 3 different fl uorescent dyes compatible with mainstream real-time termocyclers. The GAPDH detection serves as an internal control of amplifi cation and/or DNA presence in the PCR reaction. The technology is currently able to detect these high-risk genotypes of HPV: 16, 18, 31, 56. Could be adapted for further genotypes detection.
ADVANTAGES OVER EXISTING SOLUTIONS:
The main benefi t of the technology over existing diagnostic kit features resolution form of the virus (a form of free - episomal and integrated form). Integration of HPV has been associated with disease (cancer) progression. The technology is capable of absolute quantifi cation of HPV load in the sample. Able to detect only 4 copies in HPV genome in analyzed sample per PCR reaction.
DEVELOPMENT STATUS (STAGE):
Prototype of a diagnostic kit, stability tests, performance tests, primer design, optimized standard operation protocol
PUBLICATIONS:
IP PROTECTION STATUS:
TECHNOLOGY / IP OWNERS :
Palacky University Olomouc - Institute of Molecular and Translational Medicine (IMTM), Faculty of Medicine and Dentistry
More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)