Diagnostics of prostate cancer and breast cancer
INTRODUCTION:
Worldwide, there are 1.6M new prostate cancer (PCa)cases with 366 000 deaths annually. Novel early diagnostics and PCa biomarkers are needed since the best current PCa biomarker (level of prostate specific antigen (PSA) in serum) lacks specificity and accuracy for early diagnosis often resulting in mistreatment of PCa patients.
TECHNOLOGY (INVENTION) DESCRIPTION:
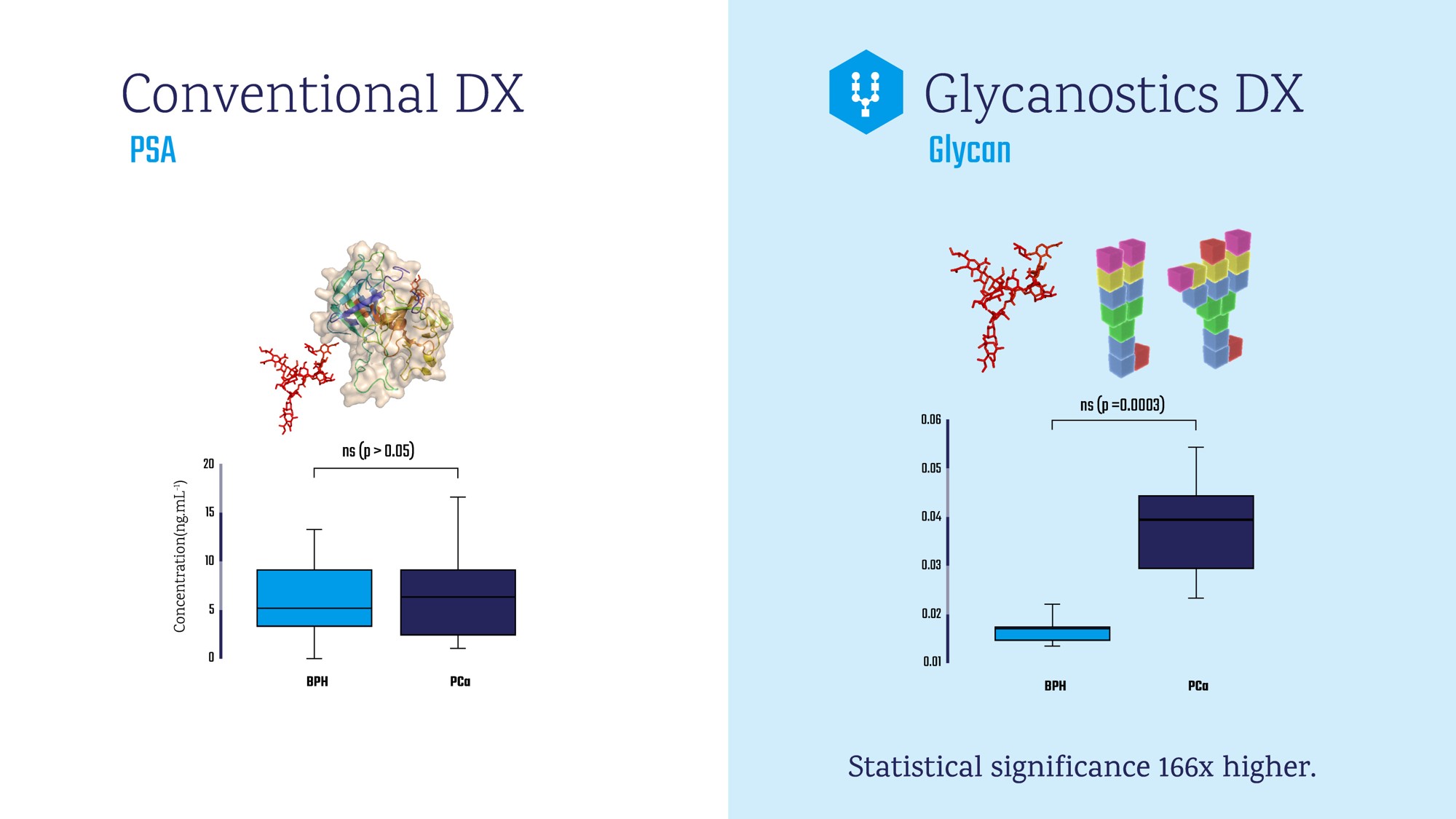
Based on a great commercial outcome from the ERC grant (311532, 2013-2017) granted to Dr. Jan Tkac (co-founder/CSO of Glycanostics), the company has developed an innovative, non-invasive, early PCa diagnostics using blood serum. Instead of quantifying PSA, the innovative PCa diagnostic assay detect glycan (complex carbohydrate) changes on PSA protein (the assay discriminates between “healthy” PSA and “sick” PSA). Our tests will be applied for identification of 1. Healthy men sent to unnecessary biopsy using current clinical practice and 2. PCa patients at early stage that are missed by the standard diagnostic procedures. This will save lives, increase life expectancy, reduce needless treatments while significantly saving costs for health-care system and society.
ADVANTAGES OVER EXISTING SOLUTIONS:
Our PCa diagnostic products relying on detection of glycan changes on PSA are unique compared to our competitors, which focus on analysis of other rather complex biomolecules such as proteins, mRNA and circulating DNA. We identified our competitors such as Beckman Coulter, OPKO Health, Hologic Inc., Exosome Diagn., Univ. Michigan and Chronix Biomed. Furthermore, we have a competitive advantage over competitors in terms of cost-effectiveness, assay accuracy & simplicity and high-throughput. Furthermore, we see opportunity to develop home tests since our simple and flexible technology allows it.
DEVELOPMENT STATUS (STAGE):
We are currently at the stage of clinical validation and at the beginning of a certification process.
PUBLICATIONS:
The technology is covered by two EPO patent applications filed in 2018: Bertok T, Tkac J (2018) Means and methods for glycoprofiling of a protein. EP 18163899.0. Tkac J, Bertok T, Klocker H (2018) Diagnosis of prostate cancer by glycoprofiling. EP 18171987.3. There are publications relevant to invention: Pihikova, D.; Pakanova, Z.; Nemcovic, M.; Barath, P.; Belicky, S.; Bertok, T.; Kasak, P.; Mucha, J.; Tkac, J. Proteomics 2016, 16, 3085. Pihikova, D.; Kasak, P.; Kubanikova, P.; Sokol, R.; Tkac, J. Analytica Chimica Acta 2016, 934, 72. Pihikova, D.; Belicky, S.; Kasak, P.; Bertok, T.; Tkac, J. Analyst 2016, 141, 1044. atd.
IP PROTECTION STATUS:
Two patent applications were filed in March 2018 (EP 18163899.0) and in May 2018 (EP 18171987.3).
TECHNOLOGY / IP OWNERS :
Both patent applications are owned by the company Glycanostics Ltd.

More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)