EpiIntestinal – a reconstructed human tissue model for simulating drug kinetics and toxicity in small intestine
INTRODUCTION:
Drug development is a complex process that depends on reliable and reproducible preclinical tests as new drugs often fail due to the inaccurate predictions in preclinical stage. Currently used in vitro models of gastrointestinal tract (GIT) often lack many properties of normal human intestinal barrier. We have recently developed a 3D reconstructed model of human small intestine consisting of primary human cells. This tissue closely resembles a morphology and physiology of human small intestine. It mimics the barrier properties and expression levels of key genes involved in drug metabolism and transport of its in vivo counterpart. The experiments aimed at modeling of drug absorption revealed that it outperforms older models. It is also capable to predict toxicity of certain drugs with better accuracy than animal models. In conclusion, these microtissues are a promising tool for predicting drug safety and bioavailability.
TECHNOLOGY (INVENTION) DESCRIPTION:
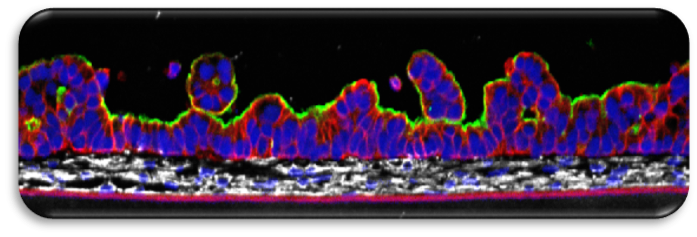
We have years of experience with bioengineering various models of human epithelia to morphologically and physiologically resemble the normal human tissues. Once established, the reconstructed tissues can be produced with very high consistency, allowing reproducibility of the results obtained over a long time. The tissues are used in various areas, such as toxicology (risk assessment), cosmetics, medical device testing, studying the inflammatory response, etc. Some of these methods are accepted by OECD. The model presented here was designed to model complex processes occurring in gastrointestinal tract. It provides morphology resembling the structure of normal small intestine epithelium with villi-like structures, brush border and presence of various functional types of cells that are normally present in vivo and can be used to model complex processes occurring in the gastrointestinal tract.
ADVANTAGES OVER EXISTING SOLUTIONS:
There are multiple in vitro models of small intestine available at the moment, including immortalized cells monolayers, artificial membranes, primary cell-based organoids and intestinal explant. All of them are capable to model certain processes, but there are known limitations in each of these, such as unphysiological barrier function or improper gene expression. Spherically shaped organoids do not provide proper access to luminal and basolateral surface, donor explants are short lived. In contrast, EpiIntestinal has normal levels of gene expression and morphology that not only mimics intestinal epithelium structure, but also allows proper observation of substance transport from both luminal and basolateral surface. The longevity of this model (~1 month) allows observing processes over time.
DEVELOPMENT STATUS (STAGE):
Fully validated, partners to perform validation studies to establish protocols accepted by regulatory bodies needed.
PUBLICATIONS:
1. 1. Ayehunie S et al., Human Primary Cell-Based Organotypic Microtissues for Modeling Small… Pharm Res. 2018 Feb 23;35(4):72. 2. Peters MF et al., Human 3D Gastrointestinal Microtissue Barrier Function As a Predictor... Toxicol Sci. 2019 Mar 1;168(1):3-17. 3. Maldonado-Contreras A et al., Shigella depends on SepA to destabilize the intestinal epithelial integrity... Gut Microbes. 2017 Nov 2;8(6):544-560. 4. Anselmo AC et al., A heat-stable microparticle platform for... Sci Transl Med. 2019 Nov 13;11(518). 5. Royston L et al., Viral chimeras decrypt the role of enterovirus .... PLoS Pathog. 2018 Apr 9;14(4):e1006962.
IP PROTECTION STATUS:
Developed and owned by MatTek Corporation, Ashland, MA
TECHNOLOGY / IP OWNERS :
Developed and owned by MatTek IVLSL, SK
More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)