Laboratory device for ultrasound specimen irradiation
INTRODUCTION:
Several signifi cant demands are placed on devices analyzing effect of ultrasound energy on various live and nonlive samples. They involve effective energy transfer, uniform distribution of ultrasound intensity with its precise value in the sample, sample manipulation, and monitoring of physical conditions, such as position, temperature, pressure,
TECHNOLOGY (INVENTION) DESCRIPTION:
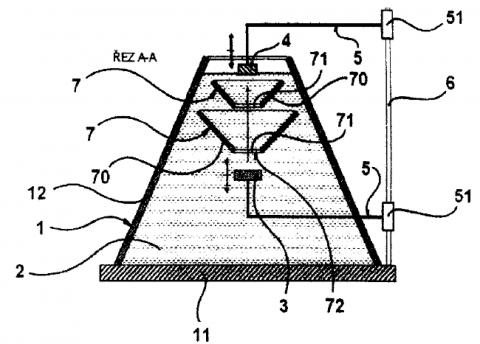
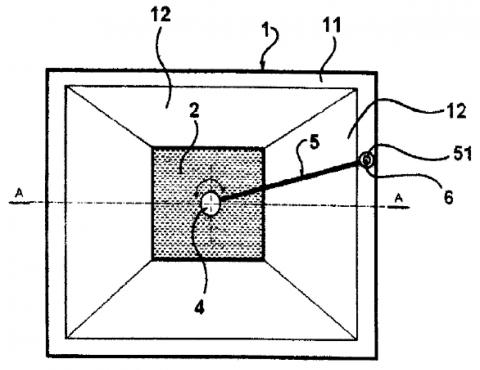
Ultrasound irradiating system composed of tank fi lled by immersion liquid and ultrasound source positioned inside the tank. Irradiated sample is fi xed by a holder in the ultrasound beam axis. The principle effect is based on barring unwanted ultrasound wave interference by nonparalel confi guration of the tank walls to the ultrasound beam axis and effi ciency may be increased by use of shielding diaphragm(s).
ADVANTAGES OVER EXISTING SOLUTIONS:
Device for specimen irradiation by ultrasound consists of tank, ultrasound source placed inside, and a sample holder in the axis of the ultrasound beam. At least one side tank wall is nonparallel with the axis of the ultrasound beam and at least one special shielding aperture is placed between source and sample. This part is in a shape of truncated cone, and arranged with its smaller base towards the source.
DEVELOPMENT STATUS (STAGE):
Prototype. Validation studies.
PUBLICATIONS:
Kolarova, H., Tomankova, K., Bajgar, R., Kolar, P., Kubinek, R. Photodynamic and Sonodynamic Treatment by Phthalocyanine on Cancer Cell Lines. Ultrasound in Medicine & Biology, 35:8, Pages 1397-1404, 2009. Tomankova, K., Kolarova, H., Kolar, P., Kejlova, K., Jirova, D. Study of cytotoxic effect of photodynamically and sonodynamically activated sensitizers in vitro. Toxicology in Vitro, 23:8, Pages 1465-1471, 2009.
IP PROTECTION STATUS:
Patent protection: CZ 19375
TECHNOLOGY / IP OWNERS :
Palacky University Olomouc - Institution of Molecular and Translational Medicine (IMTM), Faculty of Medicine and Dentistry.
More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)