Method for determining the sensitivity of patients towards the cancer treatment by HER family (namely EGFR and HER-2) inhibitors.
INTRODUCTION:
The human genome revolution brings with it large quantities of new molecular information and has spawned impressive high-throughput analytical technologies. This knowledge provides new research tools and raises the possibility of developing novel therapeutics and disease biomarkers that diagnose and treat each patient as an individual. Such an oppo
TECHNOLOGY (INVENTION) DESCRIPTION:
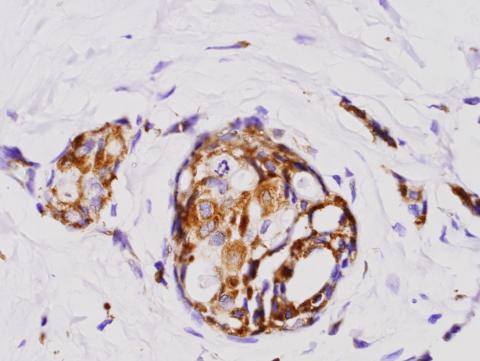
A method for determining the sensitivity of patients towards the cancer treatment by HER family (namely EGFR and HER-2) inhibitors is provided, using a new biomarker, the expression of which highly correlates with progression-free survival and overall survival in HER positive tumors, particularly of breast, colorectal, lung, pancreatic, head and neck, brain, prostate or skin. The method is based on analysis of posttranslational modifi cations of S6 ribosomal protein and can be carried out on a tumor bioptic sample or on a sample of body liquid using immunochemistry, mass spectrometry and/or other analytical tools.
ADVANTAGES OVER EXISTING SOLUTIONS:
The biomarker is frequently expressed in patient population and allows for a quick and reliable distinction between the patients benefi ting from the HER targeted therapies and the patients for whom this medication would not bring a positive effect and which can then be indicated for other, more effective therapies.
DEVELOPMENT STATUS (STAGE):
Laboratory scale, extensive validation study on patient tissues.
PUBLICATIONS:
IP PROTECTION STATUS:
Patent protection: CZ 302709 US 8,465,936 JP 5295268 EP 2 241 890
TECHNOLOGY / IP OWNERS :
Palacky University Olomouc – Institute of Molecular and Translational Medicine (IMTM), Faculty of Medicine and Dentistry Masaryk Memorial Cancer Institute in Brno
More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)