New approach to the safer pharmaceutical treatment of IBD and nephrotoxicity of 5-aminosalicylates
INTRODUCTION:
5-ASA and its derivatives are anti-inflammatory drugs indicated for the treatment of IBD. The number of IBD patients has been increasing in incidence and prevalence. Available data on serious adverse effects progressing to end-stage renal failure consist of clinical observation and a small, but increasing number of published case reports.
TECHNOLOGY (INVENTION) DESCRIPTION:
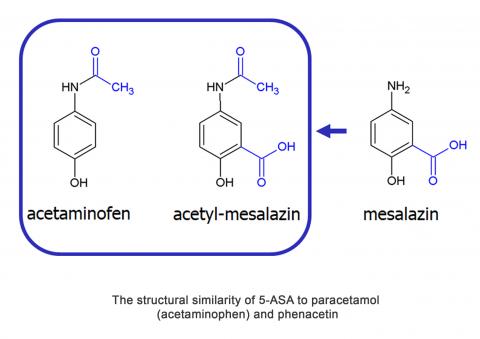
The structural similarity of 5-ASA to paracetamol (acetaminophen) and phenacetin, drugs with well documented nephrotoxic potential, and the nephrotoxicity of 5-ASA in preclinical animal models, lend further support to the hypothesis that 5-ASA does initiate the interstitial nephritis, papillary necrosis and nephrotoxicity of unknown origin. Our results have shown that detoxification products of acetaminophen metabolism and the compounds with structure similar to acetaminophen are able to significantly induce decrease of cell viability (human hepatocellular cell and renal proximal tubular cell models were used). Protection against production of these (nephro-)toxic compounds has been proven with promising results.
ADVANTAGES OVER EXISTING SOLUTIONS:
More than 88% of all UC patients receive treatment with 5-ASA in USA as well as in Europe. A number of reports have linked oral 5-ASA therapy to chronic tubulo-interstitial nephritis and this relationship is now well established. Whilst the incidence of this adverse event in the population of patients with inflammatory bowel disease is low, the morbidity in an affected individual is high with some cases progressing to end-stage renal failure requiring the dialysis or transplantation. It is necessary to develop the safer drug combination which would minimize the adverse effects described and the key patient benefits of the 5-ASA for the lifelong maintenance therapy could prevail.
DEVELOPMENT STATUS (STAGE):
Data from in vitro assays, cell toxicity studies available.
PUBLICATIONS:
IP PROTECTION STATUS:
Patent application has been in preparation.
TECHNOLOGY / IP OWNERS :
University of Pardubice
More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)