PlasmaVet, Generator of low-temperature plasma
INTRODUCTION:
Low-temperature plasma technology and its use in “plasma medicine” is a rapidly developing interdisciplinary field. Due to its bactericidal properties, cold plasma represents an effective tool for various procedures in medicine, particularly in the treatment of acute or chronic wounds, such as diabetic foot ulcers, burns and other skin pathologies.
TECHNOLOGY (INVENTION) DESCRIPTION:
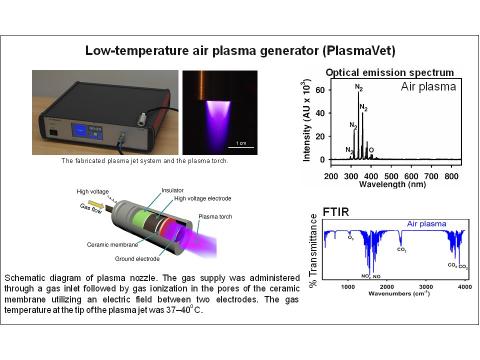
PlasmaVet represents an innovative design of low-temperature plasma source based on the atmospheric air and gas temperature 37 °C. The generator holds ES Declaration of Conformity, ITC Test Report, and it is certified as a veterinary technical device. It meets requirements such as uniform intensity distribution, magnification of the treated area, and minimizing the risk to the patient. The great advantage of this technology is also the ability to work with several different gases at the same time. Generally, plasma composition includes excited particles, such as electrons, ions, reactive oxygen and nitrogen species, which have deleterious impact on bacterial membranes and results in a rapid and non-specific bacterial damage without any harmful effects on the skin or living tissues.
ADVANTAGES OVER EXISTING SOLUTIONS:
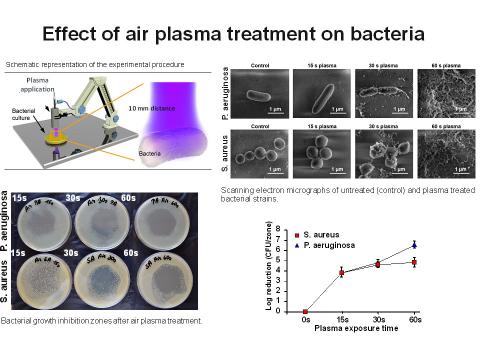
The low-temperature plasma represents a unique way how to effectively kill bacteria. Results from both preclinical and clinical studies in human medicine show that plasma treatment is safe, eliminates bacterial contamination and speeds up the healing process. As an alternative to pharmacological treatment, low-temperature plasma treatment provides the benefit of simple, cheap and nonspecific bactericidal effects without the risk of developing bacterial resistance. It also kills multiresistant bacterial strains, which represent a big health care problem in the world. The unique design is compact and easily transportable and allows comfortable patient treatment both in the room and outdoors.
DEVELOPMENT STATUS (STAGE):
CE marking, certified as veterinary technical device, preclinical studies, clinical study in preparation, scale-up ready
PUBLICATIONS:
Kubinova et al. Non-thermal air plasma promotes the healing of acute skin wounds in rats, Sci Rep. 2017;7:45183. Lunov e al. Chemically different non-thermal plasmas target distinct cell death pathways, Sci Rep. 2017;7:600. Kubinová et al. Vet. lékař. 2016; 14(2):69-75. Lunov et al. Towards the understanding of non-thermal air plasma action, RSC ADV. 2016; 6:25286. Lunov et al. The interplay between biological and physical scenarios of bacterial death induced by non-thermal plasma, Biomaterials. 2016; 82: 71-83. Lunov e al. Appl Phys Lett. 2015, 106, 053703. Lunov et al. Sci. Rep. 2014;4:7129. Kubinová et al. Léčba ran, 2016; 4, 22
IP PROTECTION STATUS:
P 304814, P 306217 Utility pattern: 31034, 29236, 29159, 27679, 25959, 23746 http://hdl.handle.net/11104/0269666.
TECHNOLOGY / IP OWNERS :
The technology/intellectual property owners are the Institute of Physics and the Institute of Experimental Medicine of the CAS.
More information
More information is available upon signing a CDA / NDA (Confidential Disclosure Agreement / Non-Disclosure Agreement)